
The Importance of Workplace Wellness Programs
Buying fresh bananas a couple times a week for your employees costs too much. You already spend enough on their healthcare. Besides, they can go to the vending machine if they want a snack. Let’s step back for a second though. Bananas cost about a dollar for six employees. An employer will lose an […]
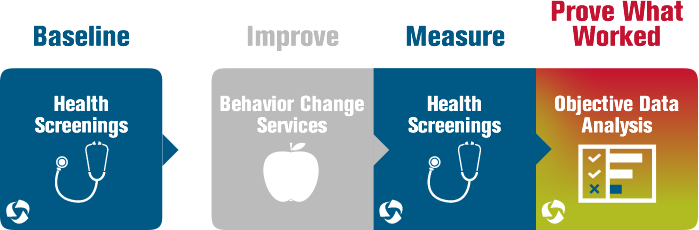
Making a Successful Investment in Workplace Wellness
Despite the fact that companies of any size can achieve remarkable ROI with a sound workplace wellness program in place, many smaller healthcare groups continue to miss out on the benefits. According to a Worksite Health Promotion Survey, larger companies were more likely to offer a benefit in almost every category of health–promoting programs or […]
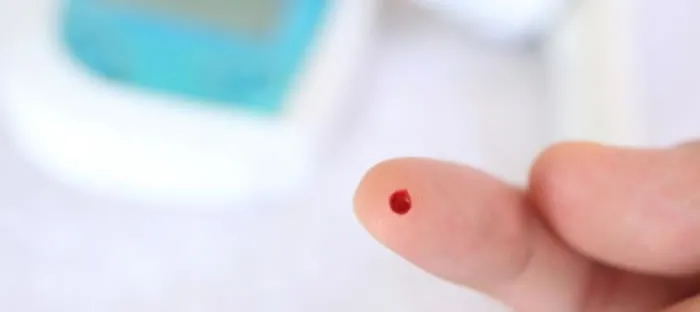
Feds say “No” to finger-stick A1c: Health Screening Compliance
A few months ago, we were engaged in an RFP process for one of our most valued municipal clients of over 3 years. As part of that process, our client asked us to provide pricing for “point of care” (a.k.a. finger-stick) Hemoglobin A1c testing. As we began to explain that, practically speaking, there is no […]
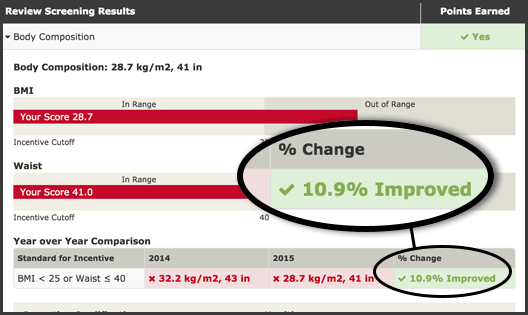
Outcomes Based Percent Improvement Thresholds: More Than Just a “Reasonable Alternative”
We all know the regulations require outcomes based wellness incentive program design to incorporate what are called “reasonable alternatives.” Typical examples include a physician exemption of some kind and one or two different activity requirements such as completing an online health education class or speaking with a health coach by phone two times or more. […]

Finger-stick A1c: Compliance Related Documents
Since our first communication regarding point-of-care A1c screenings NOT being compliant with CLIA, we have had dozens of calls with concerned consultants, brokers & large employers, many of whom are now making the correct choice and switching away from fingerstick A1c. The primary point of interest from these calls has been what documentation we can provide […]

Is Your Wellness Portal ADA Compliant?
People with disabilities make up about one-fifth (20%) of the population of the United States. Not all of these people have disabilities that make it difficult for them to access the internet, but a significant portion does. For many public institutions, creating a website that is compliant with Section 508 Accessibility Standards is the law. […]